
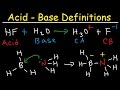
















So our conjugate base is stabilized by resonance, and since our conjugate base is stabilized by resonance, that means acetic acid is more likely to donate to this proton, and that's why we see a lower pKa value. If we compare that to the conjugate base for ethanol, alright this is called the ethoxide anion, alright we can't draw a resonance Its conjugate acid is H 2 CO 3, and its conjugate base is CO 3 2–. The use of conjugate acid-base pairs allows us to make a very simple statement about relative strengths of acids and bases. The stronger an acid, the weaker its conjugate base , and, conversely, the stronger a base, the weaker its conjugate acid. 3) Identify the acid, base, conjugate acid, and conjugate base of HSO4 + NH3 -----> SO4 + NH4 Acid: HSO4 Base: NH3 Conjugate acid: NH4 Conjugate base: SO4 Got the wrong answer? Click Here to review Click Here to go back to the QUIZ!! 4) Identify the acid, base, conjugate acid, and conjugate base of C2H3O2 + HCl -----> C2H4O2 + Cl Acid: HCL Base The conjugate base of a strong acid is a very weak base, and, conversely, the conjugate acid of a strong base is a very weak acid. Figure \(\PageIndex{1}\) The Relative Strengths of Some Common Conjugate Acid–Base Pairs. The strongest acids are at the bottom left, and the strongest bases are at the top right. The conjugate base of a strong Calculate the degree of ionisation and p H of 0. 0 5 M solution of a weak base having the ionization constant (K c ) is 1. 7 7 × 1 0 − 5. Also calculate the ionisation constant of the conjugate acid of this base. Definition of Conjugate Acid Base Pair. Conjugate acid base pair or protonic definition of acids and bases independently proposed by Bronsted and Lowery in 1923 for learning chemistry or chemical science. According to this definition, an acid is any hydrogen atom containing material (molecule or ion) that can release a proton or hydrogen ion to any other substances, whereas a base is any A more general definition is that a conjugate base is the base member, X-, of a pair of compounds that transform into each other by gaining or losing a proton. The conjugate base is able to gain or absorb a proton in a chemical reaction. The conjugate acid donates the proton or hydrogen in the reaction. A conjugate base is what's left over after an acid loses a hydrogen ion. In general, conjugate bases are negatively charged and have higher pH values. Every acid has a conjugate base. The conjugate base of bicarbonate, HCO 3- is carbonate, CO3 2-.. HCO3- is a conjugate acid, H 2 CO 3 A conjugate base contains one less H atom and one more - charge than the acid that formed it. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions. HCO₃⁻ + H₂O → H₂CO₃ + OH⁻. base + acid → Conj A + Conj B. We see that HCO₃⁻ becomes H₂CO₃.
[index] [9972] [9469] [8794] [6344] [5228] [923] [7420] [8144] [8390] [3434]
Conjugate acids and bases are usually introduced in organ... Skip navigation Sign in. Search. Loading... Close. This video is unavailable. Watch Queue Queue. Watch Queue Queue. All this talk of conjugate sounds scary! Not really, this video looks at what a conjugate base and acid are with multicoloured equations. Take a look to find... Donate here: http://www.aklectures.com/donate.php Website video: http://www.aklectures.com/lecture/conjugate-acid-base-pairs Facebook link: https://www.faceb... +2 pts per boxconjugate base stabilization increases acid strength Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is... This chemistry video tutorial explains the concept of acids and bases using the arrhenius definition, bronsted - lowry and lewis acid base definition. It al... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... For astonishing organic chemistry help: https://chemistrybootcamp.com/To see my new Organic Chemistry textbook: https://tophat.com/marketplace/science-&-math... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Copyright © 2024 hot.onlinerealtopmoneygames.xyz